JCCP launches Botulinum Toxin complications study
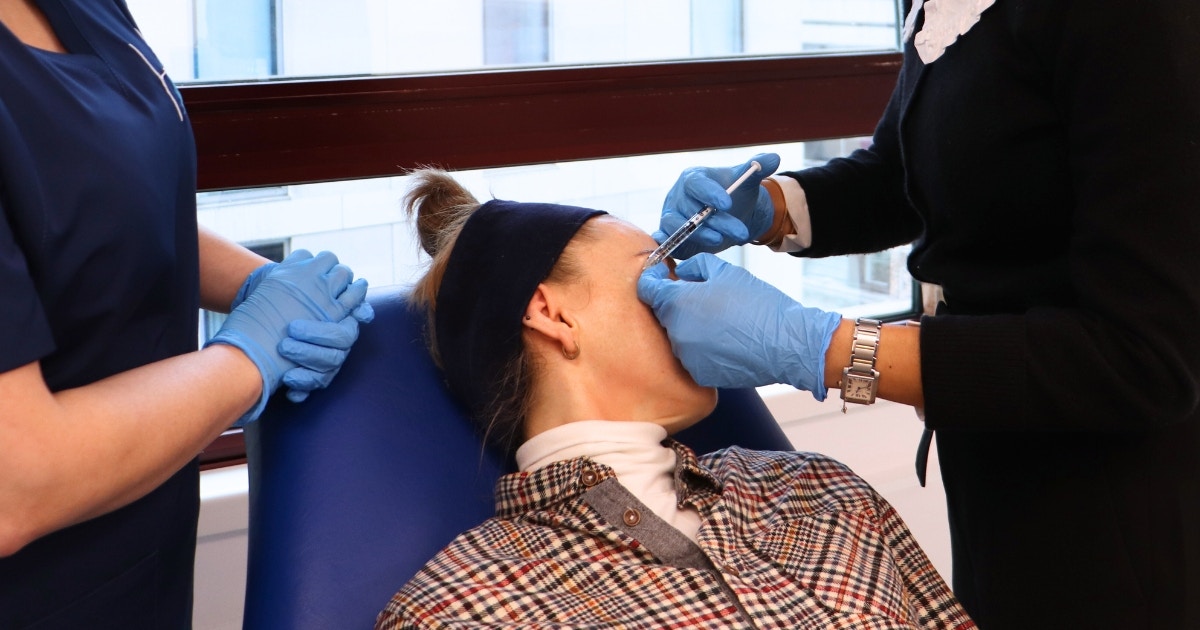
A new botulinum toxin complications study has been announced by the Joint Council of Cosmetic Practitioners (JCCP). The UK industry body is known for its work towards improving safety standards of aesthetic practise.
This study will be a collaboration between the JCCP, the University College London (UCL) and the British Association of Aesthetic Plastic Surgeons (BAAPS).
In a JCCP press release dated 17 January 2023, the aim of this joint study into botulinum toxin complications is to build “a scientific evidence base to inform safer evidence-based practice”.

SUBMITTING EXPERIENCES OF BOTULINUM TOXIN COMPLICATIONS
In order to better understand patients’ experiences, the JCCP has advised the following…
“This novel survey has a primary aim of capturing individual consumer experiences following receipt of botulinum toxin injections. The survey was created with a cross-disciplinary team including researchers, psychologists, clinicians, and patient representatives. If you have had a difficult or challenging experience that you wish to share, this survey will provide the platform for you to do so.”
Patients can submit their first-hand accounts of botulinum toxin complications by completing an online form. The JCCP also notes that this research is “particularly timely” given the “highly anticipated government public consultation on the design of a new licence for non-surgical cosmetic practice in England”.
The press release notes that the Department of Health and Social Care are due to launch this in the spring of 2023. As such, the JCCP hope that the survey data will “inform the public consultation”. They also hope it will “assist the UK Government in its endeavour to introduce statutory regulation for the aesthetic and non-surgical cosmetic industry.”

BOTULINUM TOXIN COMPLICATIONS UNDER-REPORTED TO THE MHRA
A team of UK researchers obtained and evaluated data from the Medicines and Healthcare products Regulatory Agency (MHRA) on the adverse effects of botulinum toxin (BoNT-A). This happened in 2020 and they were the first to do so.
Their findings were published in the Journal of Plastic, Reconstructive and Aesthetic Surgery in June 2021. These demonstrated what the study authors believed to be an underestimation “of the adverse events of aesthetic BoNT-A treatment” by the MHRA. They felt these “would have implications for patient safety and informed consent”.
As such, they proposed that tougher regulations be introduced into the UK aesthetics industry.
An article published in The Guardian newspaper about this research estimated that 900,000 botulinum toxin injections were performed each year in the UK. As the neurotoxin’s cosmetic effects continue to grow in scope as well as popularity, it’s probable that this number has increased since then.
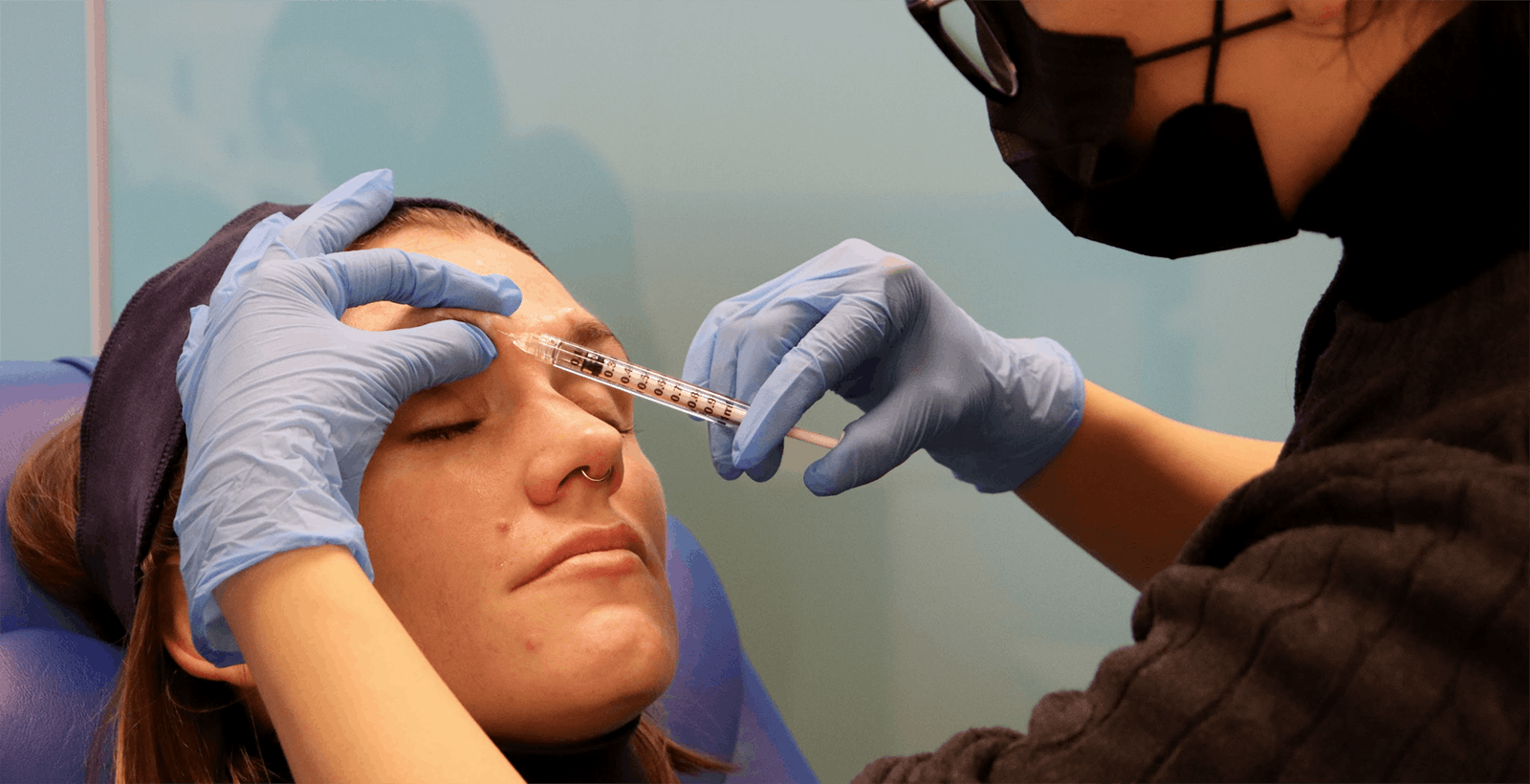
BEST PRACTICE BOTOX TRAINING FOR HEALTHCARE PROFESSIONALS
Safe, effective botulinum toxin administration, requires excellent botox training. At Harley Academy we offer a range of aesthetic medicine courses to healthcare professionals. Each of these includes experience-specific botox training modules and practical injecting under the guidance of a mentor.
Whether you’re a beginner or a more advanced injector, if you’re a doctor, dentist, nurse or midwife, we have courses that’ll provide you with a solid foundation for your botulinum toxin practice.
Botox complications such as spocking and ptosis can happen to us all. However, with a thorough grounding in how to prevent and manage these issues, you can minimise the risk. You’ll also be prepared to safely and competently deal with these issues by knowing how to correct them, where possible.
If you’d like advice on the best botox training options for you, contact our Courses team today. Alternatively, you can download our Prospectus to check out our full range of aesthetics courses.
All information correct at the time of publication.
Download our full prospectus
Browse all our injectables, dermal fillers and cosmetic dermatology courses in one document
By submitting this form, you agree to receive marketing about our products, events, promotions and exclusive content. Consent is not a condition of purchase, and no purchase is necessary. Message frequency varies. View our Privacy Policy and Terms & Conditions
Attend our FREE open evening
If you're not sure which course is right for you, let us help
Join us online or in-person at our free open evening to learn more
Our Partners














STAY INFORMED
Sign up to receive industry news, careers advice, special offers and information on Harley Academy courses and services

